Trad: Il sale addensa riducendo la densità di carica micellare, contribuendo a promuovere la conversione delle micelle sferiche in micelle a forma di bastoncino. Storicamente è stato utilizzato il cloruro di sodio. Tuttavia, i sali bivalenti come il solfato di magnesio sono più efficienti se compatibili nella formulazione. La viscosità delle formulazioni addensate con sale diminuisce con l'aumentare della temperatura e non stabilizzerà le particelle sospese. L'uso di una quantità eccessiva di sale (>2%) può anche influire sulla limpidezza e sul punto di intorbidimento della formulazione.Salt thickens by reducing micelle charge density, helping to promote the conversion of spherical micelles to rod-shaped micelles. Historically, sodium chloride has been used. However, divalent salts like magnesium sulfate are more efficient if compatible in the formulation. Viscosity of salt-thickened formulations decreases with increasing temperature and will not stabilize suspended particles. Using too much salt (>2%) can also impact the clarity and cloud point of the formulation.
Cavolo! Ho trovato una curva di addensamento del sarcosinato (che come è noto addensa a pH5) e il discrimine tra denso e liquido è sempre dato dalle micelle che diventano a bastoncino e non più tonde:
https://www.sciencedirect.com/science/a ... 5720300169
Abstract
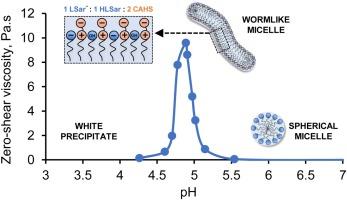
Wormlike micelles in mixed surfactant mixture containing an amino acid-derived anionic surfactant, sodium lauroyl sarcosinate (SLSar), and a zwitterionic surfactant, cocoamidopropyl hydroxysultaine (CAHS) were investigated. The total surfactant concentration was maintained at 15% wt/v, while the ratio of SLSar and CAHS was varied and the pH was lowered from 7.5 to 4.5. Steady-shear rheology, potentiometric titration, cross-polarized microscopy, zeta potential and cryo-TEM were used to obtain zero-shear viscosity and analyze the structure-rheology relationship. As pH dropped, viscosity increased to a maximum followed by a viscosity decrease in four out of five tested systems. The synergistic effect was strongest in the SLSar 6 %:CAHS 9 % composition or an equivalent molar ratio of 1:1 between the two surfactants, for which the viscosity maximum was 9.6 Pa.s at pH 4.8. The viscosity peak of each system occurred in the pH range 4.8–5.0, close to the system’s experimental pKa. The intermolecular forces between the SLSar acid form, SLSar anionic form and CAHS reduced the charge repulsion and contributed to the thickening behavior of these systems.